HeLa-cells
HeLa cells have been used in research since the 1950s. This is an immortal cell line, which is very well suited for testing the polio vaccine, for example. The cell line is now commercially distributed and used for many experiments. Thousands of patents pending worldwide are based on scientific findings from experiments with HeLa cells.
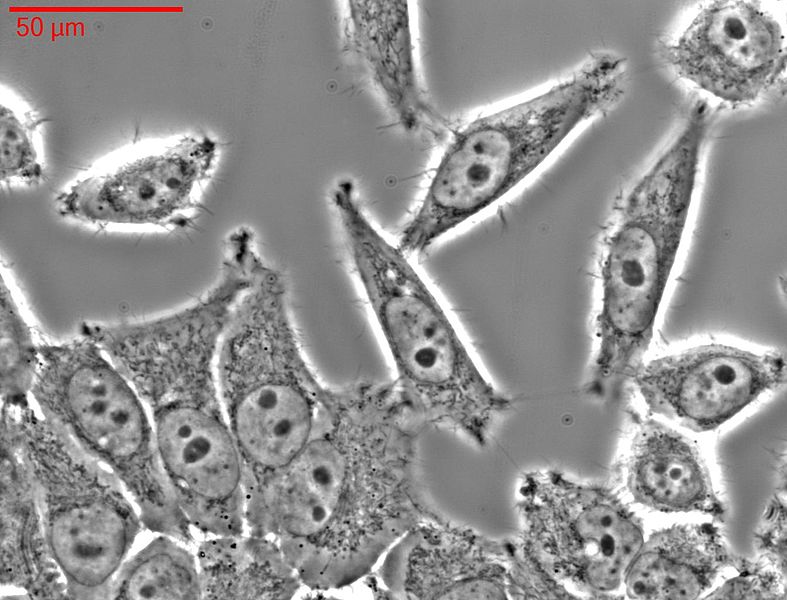
Photo: HeLa-cells in light microscops
Source: Fraunhofer IBMT - Anastasiadis, Weiß/Wikimedia
There is a special story behind this cell line and its name: Henrietta Lacks was a patient at Johns Hopkins Hospital in Baltimore. In 1951 she was treated there for a cervical tumor. The Johns Hopkins Hospital was one of the few hospitals that also treated African American patients. Often, tacit consent was assumed for participation in studies. Henrietta Lacks' gynecologist, Howard W. Jones, removed a cell sample from the tumor. He handed it over to cell researcher George Otto Gey, who developed the potentially immortal HeLa cell line. Based on the name of the patient Henrietta Lacks, he called them HeLa cells. Henrietta Lacks was never informed about the use of her cells. Even her family only learned years later of the use of the cells. Excerpts from Henrietta's patient file were published without the family's consent. In 2013, her genome sequence was decrypted and included in a freely accessible database. This triggered a global debate. Only thereafter was an agreement arrived at with Henrietta Lacks' descendants, which regulates the use of the data. Two family members today have seats in a committee that decides on access to the DNA code.

Photo: Plaque for Henrietta Lacks in Lacks in Clover, Virginia
Source: Emw/Wikimedia
Informed Consent
Patients must consent to medical treatments. This includes the use of their cells for research. By informed consent we mean that a patient is in a condition to be able to make the decision for themselves. The legal term for this is the capacity to consent. In exceptional cases, a proxy may also give this consent. The scientist also has a duty to inform. Only when the patient has been informed, can he or she give an informed consent. Informed consent emerged as early as the beginning of the 20th century as an ethical research principle. It has nevertheless been repeatedly violated.
It was not until 1964 that the World Medical Association incorporated informed consent in its ethical principles for medical research on humans at its General Assembly in Helsinki – not least because of the atrocities committed by Nazi doctors on their Jewish prisoners. The document is therefore known as the Helsinki-Declaration. There is still some disagreement about whether it is possible to fully inform the patient and how the capacity to consent is defined in detail.